Project P8
P8: Receiver Architectures for Information Recovery from Airborne MC Signals
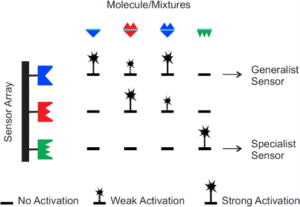
Motivation and state of the art:
Various receiving strategies have evolved in nature to capture airborne molecular information, ranging from a specific endowment with receptors to differently shaped antennae, or certain behaviours1-5. In nature, living organisms do not use their olfactive organs as static entities but rather orient and move their “detective tools” versus and within the odor plume to facilitate detection, discrimination, and localization. The biology of mammalian and insect olfactory systems6-8, sampling, sniffing, and olfactive organ movement (e.g., back and forth, turning)9,10 have been extensively studied in the literature. Similarly, the development of technical systems that allow for the detection of odors is an active field of research5. Currently available technical solutions for the detection of airborne chemicals include electrical sensors, gravimetric sensors, optical sensors in combination or not with gas chromatographic, mass spectrometric, and spectroscopic systems, and biosensors11. To enhance the performance of such systems in terms of sensitivity and selectivity, mainly material development12 in view of technologies for pre-separation or pre-concentration of volatiles13 [has been considered. However, the theoretical and experimental impact of different reception architectures as well as active movement and “smell sampling behavior” on the performance of synthetic airborne MC has not been well investigated yet, and is currently not accounted for in respective technical systems.
Objectives:
The goals of this project include (i) investigation of various sensing architectures (including different types of sensing elements, geometries, and movement-aided mechanisms) inspired by natural receptive systems, (ii) evaluation of their usefulness for synthetic airborne MC systems, and (iii) analysis of the impact of the receiver architecture on robust recovery of odor objects against potential variations of their composition caused during release and propagation.
References
1.Hansson and M. Stensmyr, „Evolution of Insect Olfaction,“ Neuron,vol. 72, pp. 698-711, Dec. 2011.
2.B. Vosshall, H. Amrein, P. Morozov, A. Rzhetsky, and R. Axel, „A Spatial Map of Olfactory Receptor Expression in the Drosophila Antenna,“ Cell,vol. 96, pp. 725-736, Mar. 1999.
3.Pelosi, I. Iovinella, J. Zhu, G. Wang, and F. Dani, „Beyond Chemoreception: Diverse Tasks of Soluble Olfactory Proteins in Insects,“ Biol. Rev., vol. 93, pp. 184-200, May 2018.
4.Rokni, V. Hemmelder, V. Kapoor, and V. Murthy, „An Olfactory Cocktail Party: Figure-Ground Segregation of Odorants in Rodents,“ Nat. Neurosci., vol. 17, pp. 1225-1232, Aug. 2014.
5.M. Koehl, „The Fluid Mechanics of Arthropod Sniffing in Turbulent Odor Plumes,“ Chem. Sens., vol. 31, pp. 93-105, Feb. 2006.
6.Ache and J. Young, “Olfaction: Diverse Species, Conserved Principles,” Neuron, vol. 48, pp. 417-430, Nov. 2005.
7.Zhao and C. McBride, „Evolution of Olfactory Circuits in Insects,“ J. Comp. Physiol. A: Neuroethol. Neural Behav. Physiol., vol. 206, pp. 353-367, Jan. 2020.
8.Mori and H. Sakano, “Olfactory Circuitry and Behavioral Decisions,” Annu. Rev. Physiol., vol. 23, pp. 231-256, 2021.
9.Beauchamp, M. Scheibe, T. Hummel, and A. Buettner, „Intranasal Odorant Concentrations in Relation to Sniff Behavior,“ Chem. Biodivers., vol. 11, pp. 619–638, Apr. 2014.
10.J. Birgiolas, et al., „SwarmSight: Real-Time Tracking of Insect Antenna Movements and Proboscis Extension Reflex using a Common Preparation and Conventional Hardware,“ J. Vis. Exp.: JoVE, pp. 1-11, e56803, Dec. 2017.
11.David Morgan, „Trail Pheromones of Ants,“ Physiol. Entomol.,vol. 34, pp. 1-17, Feb. 2009.
12.K. Shimizu and C.Stephenson, “Molecularly Imprinted Polymer Sensor Arrays,” Curr. Opin. Chem. Biol., vol. 14, pp. 743-750, 2010.
13.Lima, L. Hernandez, A. Carvalho, R. Carvalho, and M. da Silva, “Corrosion Resistant and Adsorbent Plasma Polymerized Thin Film,” Actuators B Chem., vol.14, pp. 349-360, 2009.
